two solutions of the same uv absorbing molecule were analyzed|The Beer : online sales Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using 1 cm pathlength cells. webMonica, Maggy, Jimmy Five, Smudge and their friends from the Lemon Tree Street need your help on a great adventure! - 1 to 4 players. Play with friends and family sharing a couch! But, if alone, you can also enjoy our single .
{plog:ftitle_list}
Resultado da 8 de mar. de 2022 · INFLUENCER Danny Maria não tinha vontade de cantar, mas virou a chave - DIVULGAÇÃO Natural do Ibura, periferia da Zona Sul .
The Beer
Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using 1 cm pathlength cells. Use data from the table to calculate by what percentage the concentration of Solution B .Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using 1 cm pathlength cells. Use data from the table to calculate by what .
Solved Two solutions of the same UV
Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using 1 cm pathlength cells.
to examine the UV absorbing characteristics of a variety of sunscreen preparations; to use results of UV analysis to calculate concentrations of a UV absorbing compound; examine SPF .
Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using 1 cm pathlength cells. Use data from the table to calculate by what .
Chemistry. Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using 1 cm pathlength cells. Use data from the table to .
Spectrometry. The absorbance $A$ of a solution is defined as $$A=\log _{10}\left(I_{0} / I\right)$$ in which $I_{0}$ is the incident-light i. The Beer-Lambert law relates the attenuation of light to the properties of the material through which the light is traveling. This page takes a brief look at the Beer-Lambert .
VIDEO ANSWER: Beer's law states that there's a direct relationship between concentration and absorbent. If we have one solution with an absorbent of 0.35, I think it is solution A. The .
SOLVED: Two solutions of the same UV
Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using I cm pathlength cells. Use data from the table to calculate by what percentage the concentration of Solution B is compared to that of Solution A Sample Absorbance at 355 nm A 0.50 B 1.00 In regards to concentration, what percentage is sample .Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using 1 cm pathlength cells. Use data from the table to calculate by what percentage the concentration of Solution B is compared to that of Solution A. Sample A B Absorbance at 350 nm 0.35 0.95 In regards to concentration, what percentage is sample B .Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using 1 cm pathlength cells. Use data from the table to calculate by what percentage the concentration of Solution B is compared to that of Solution A. Sample Absorbance at 320 nm А 0.35 0.85 In regards to concentration, what percentage is sample B . Most UV-vis instruments can analyze solid samples or suspensions with a diffraction apparatus (Figure \(\PageIndex{7}\)), but this is not common. UV-vis instruments generally analyze liquids and solutions most efficiently. Figure \(\PageIndex{7}\) Schematic representation of the apparatus for collecting UV-vis spectra from solid materials.

Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using 1 cm pathlength cells. Use data from the table to calculate by what percentage the concentration of Solution B is compared to that of Solution A. In regards to concentration, what percentage is sample B compared to sample A? That means that in order to absorb light in the region from 200 - 800 nm (which is where the spectra are measured), the molecule must contain either pi bonds or atoms with non-bonding orbitals. Remember that a non-bonding orbital is a lone pair on, say, oxygen, nitrogen or a halogen. Groups in a molecule which absorb light are known as .The ultraviolet-visible (UV-vis) absorption spectrum of a tartrazine solution exhibits two major peaks located at 257 and 428 nm but with minimal absorption beyond ~600 nm (fig. S1, A and B). We verified the chemical composition of tartrazine used .
Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using 1 cm pathlength cells. Use data from the table to calculate by what percentage the concentration of Solution B is compared to that of Solution A. Sample Absorbance at 330 nm A 0.55 B 0.80 In regards to concentration, what percentage is sample .
If the isoprene spectrum on the right was obtained from a dilute hexane solution (c = 4 * 10-5 moles per liter) in a 1 cm sample cuvette, a simple calculation using the above formula indicates a molar absorptivity of 20,000 at the maximum absorption wavelength. Indeed the entire vertical absorbance scale may be changed to a molar absorptivity scale once this information about .
Question: Consider two different solutions of the same UV-absorbing compound. Upon UV-analysis, Solution B gave an absorbance of 1.5 and Solution A gave an absorbance of 0. 5 at the same wavelength. Solution B is higher in concentration by what ratio (or percentage)? Hint: A = e . c . lWill rate lifesaver! For example, ethanal has two absorption peaks in its UV-visible spectrum - both in the ultra-violet. One of these corresponds to an electron being promoted from a lone pair on the oxygen into a pi anti-bonding orbital; the other from a \(\pi\) bonding orbital into a \(\pi\) anti-bonding orbital.Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using cm pathlength cells. Use data from the table to calculate by what percentage the concentration of Solution B is compared to that of Solution A Sample Absorbance at 330 nm 0.55 0.80 regards to concentration, what percentage is sample B .The basic setup is the same as for IR spectroscopy: radiation with a range of wavelengths is directed through a sample of interest, and a detector records which wavelengths were absorbed and to what extent the absorption occurred. . (and sometimes in UV-vis as well). Solution. Show Answer Here is the absorbance spectrum of the common food .
Using UV-absorption spectra to help identify organic compounds. . has a maximum absorption at 171 nm. The two conjugated double bonds in buta-1,3-diene have a maximum absorption at a longer wavelength of 217 nm. We also talked about the two peaks in the spectrum of ethanal (containing a simple carbon-oxygen double bond) at 180 and 290 nm .What does an absorption spectrum look like. The diagram below shows a simple UV-visible absorption spectrum for buta-1,3-diene - a molecule we will talk more about later. Absorbance (on the vertical axis) is just a measure of the amount .
to examine the UV absorbing characteristics of a variety of sunscreen preparations; to use results of UV analysis to calculate concentrations of a UV absorbing compound; examine SPF ratings and understand its limitations and uses; to determine what components in sunscreens give better UVA absorptionThe concentrations were adjusted so that the absorption intensities of the components were roughly the same. It can be seen in Fig. 2 that peak wavelengths tend to be shifted toward the long wavelength region as the . Determine the concentration of copper(II) ions in solution using spectrophotometric analysis. Many substances absorb UV (ultraviolet) or visible light. When a substance absorbs certain frequencies (colors) of light, some of them are transmitted, and the solution appears colored.The material of cuvette should have a sufficient transmission at a given wavelength. Light attenuation on the cuvette walls should not affect the outcome of an analysis. Glass cuvettes are not used in the UV region for analysis below 370 nm as they absorb the radiation. It is recommended to use them only in the visible region.
instron tensile compression tester model 4481
Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using 1 cm pathlength cells. Use data from the table to calculate by what percentage the concentration of Solution B is compared to that of Solution A. Sample- Absorbance at 340 nm A- 0.40 B- 0.85 In regards to concentration, what .
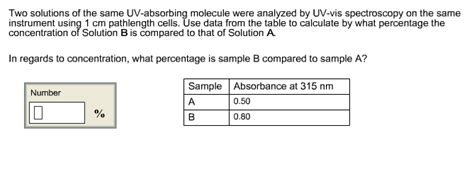
Two solutions of the same UV-absorbing molecule were analyzed by UV-vis spectroscopy on the same instrument using 1 cm pathlength cells. Use data from the table to calculate by what percentage the concentration of Solution B is compared to that of Solution A. Sample- Absorbance at 340 nm A- 0.40 B- 0.85 In regards to concentration, what . Molar absorptivity. Molar absorptivity (\(epsilon\)) is a physical constant, characteristic of the particular substance being observed and thus characteristic of the particular electron system in the molecule. Molar absorptivities may be very large for strongly absorbing chromophores (>100,000) and very small if absorption is weak (10 to 100).
Introduction. According to Beer’s Law, A = εLc, a substance’s concentration and absorbance are directly proportional under ideal conditions: a high-concentration solution absorbs more light.In comparison, a low-concentration solution absorbs less light. Beer’s Law allows you to determine an unknown phosphate concentration after determining the absorbance because .
Experiment 1: UV Analysis of Sunscreens
Beckman DU640 UV/Vis spectrophotometer. Ultraviolet (UV) spectroscopy or ultraviolet–visible (UV–VIS) spectrophotometry [1] [2] [3] refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. [2] Being relatively inexpensive and easily implemented, this methodology is widely .Multicomponent Analysis using Molecular Absorption Beer’s law is valid simultaneously for all absorbers in a solution. Let’s imagine that we have two analyte species, A and B, present in a solution. The absorbance due to the two individual species will usually add to give the total absorbance of the solution, as shown in the following figure:
Chemo 238 Sapling Questions Flashcards
You need a spectrometer to produce a variety of wavelengths because different compounds absorb best at different wavelengths. For example, p-nitrophenol (acid form) has the maximum absorbance at approximately 320 nm and p-nitrophenolate (basic form) absorb best at 400nm, as shown in Figure 3. Figure 3: Absorbance of two different compoundsThe UV absorption spectrum of 20 μg/mL mirtazapine in methanol, water, 0.1 N NaOH, and 0.1 N H 2 SO 4 were recorded using a Shimadzu UV spectrophotometer (model UV-1800) using 1-cm matched quartz cells. The absorption spectra of a reference and test solution were carried out in a 1 cm quartz cell over the range of 200–400 nm.
CHEM 238 Lab Final Flashcards
WEB30 de set. de 2021 · Contos eróticos sobre crossdresser página 48. Ensaios; Blog; Últimos; Arquivo; Rankings. Mais votados no novo ranking; Mais lidos; Autores mais ativos no último mês; Temas; Listas; Publique; . 2000-2024 - Casa dos Contos - Todos os direitos reservados. 2024-02-29 21:25:58 .
two solutions of the same uv absorbing molecule were analyzed|The Beer